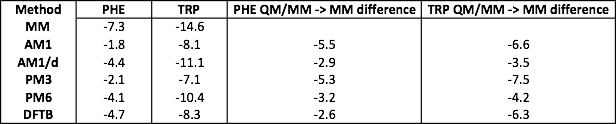
Exercise 1 Model Answer

An example table is above. Note that the more negative the energy, the stronger the interaction energy between the side-chain and water.
Question 1.1 - The interaction energies between TRP and water are consistently more negative (and thus more favourable) than those between PHE and water. This shows that TRP has a stronger interaction than PHE, which is not surprising as TRP contains a nitrogen, so is able to form hydrogen bonds with water.
Question 1.2 - AM1/d gives the strongest interaction energy for TRP, while DFTB gives the strongest interaction for PHE (AM1/d is pretty close for PHE too). None of the semi-empirical QM/MM models predict an interaction energy that is as strong as that for MM. MM gives an interaction energy between each side-chain and water that is about 3+ kcal mol-1 stronger than QM/MM.