Exercise 6 Model Answer

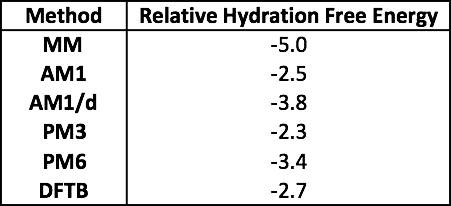
The table above shows the relative hydration free energy between PHE and TRP for all of the models (in kcal mol-1).
Question 6.1 - No matter which method you chose, the relative QM/MM hydration free energy is less negative (less favourable) than the MM relative hydration free energy. Despite this, both the MM and QM/MM relative hydration free energies are negative, which means that they both predict that TRP has a more negative (more favourable) hydration free energy than PHE and thus TRP has the greater affinity for water (i.e. TRP is more soluble than PHE).
Question 6.2 - AM1/d gives the most negative relative hydration free energy, and also gives the closest relative hydration free energy compared to MM. This suggests that, if you are looking for a QM model that is the closest match to MM, then you should choose to simulate these side-chains using AM1/d.